LIST OF PUBLICATIONS
[179]
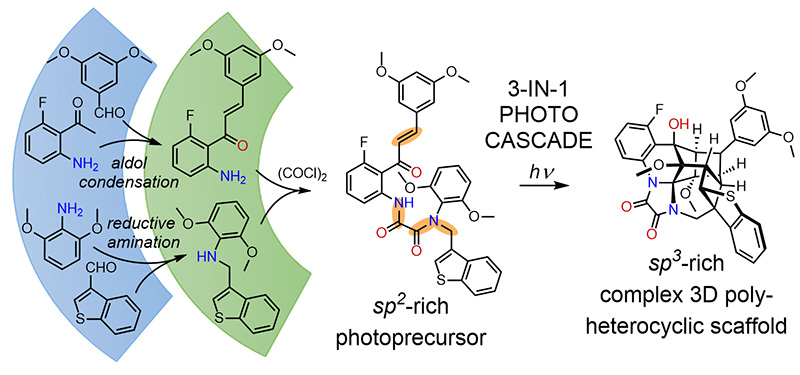
Complexity-Building Exhaustive Dearomatization of Benzenoid Aromatics within an ESIPT-Initiated Three-Step Photochemical Cascade. Beduru, S.; Huple, D.B.; Kutateladze, A.G. Angew. Chem. Int. Ed. , 2025, 64, (2), e202415176. [DOI: 10.1002/anie.202415176] 
[178]
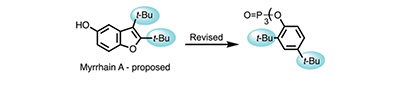
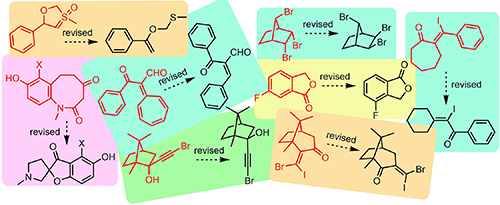
Myrrhain A: Structural Reassignment Highlights the Relevance of Plasticizer Contamination in Chemical and Bioactivity Studies. Bates, R.W.; Elyashberg, M.; Khandare, S.P.; Appendino, G.; Kutateladze, A.G.; Williams, C.M. Org. Lett., 2025, 27, (49), 13633-13636. [DOI: 10.1021/acs.orglett.5c04575] 
[177]
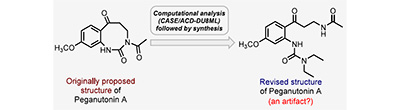
Peganutonin A: Computationally Informed Synthesis of Its Revised Structure. Saranya, N. S. S.; Choudhury, R.; Supekar, P. R.; Beduru, S.; Williams, C. M.; Elyashberg, M. E.; Kutateladze, A. G.; Bates, R. W.; Reddy, D. S. J. Nat. Prod., 2025, 88, (5), 1253-1258. [DOI: 10.1021/acs.jnatprod.5c00322] 
[176]
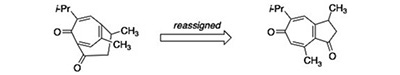
Crotonguaienone G and pernambucone are identical. Bates, R. W.; Williams, C. M.; Kutateladze, A. G.; Elyashberg, M. E. Fitoterapia, 2025, 185, 106728. [DOI: 10.1016/j.fitote.2025.106728] 
[175]
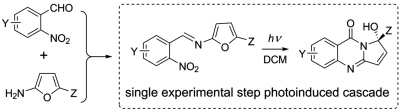
Oxidative Control of Photoinduced Cascade Electrocyclizations in Aromatic Azido Imines to Access Complex Fused Imidazoles or Pyrazoles. Reddy, D.S.; Novitskiy, I.M.; Beloglazkina, A.A.; Kutateladze A.G. Org. Lett., 2024, 26, 2558. [DOI: 10.1021/acs.orglett.4c00361] 
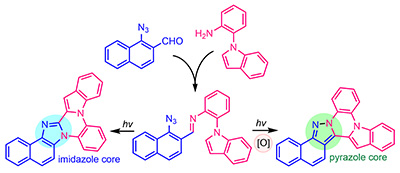
[174]
Reassignment of the Structure of Setosol. Bates, R.W.; Elyashberg, M.; Kutateladze, A.G.; Williams, C.M. Eur. J. Org. Chem., 2024, e202400431. [DOI: 10.1002/ejoc.202400431] 
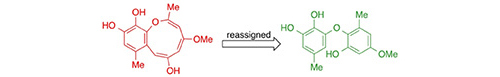
[173]
Systematic Photoassisted Access to Designer Polyheterocycles via Modular Blocks and Scaffolding. Holt. T. A.; Novitskiy, I. M.; Kutateladze, A. G. Org. Lett., 2024, 26, (3), 734-738. [DOI: 10.1021/acs.orglett.3c04186] 
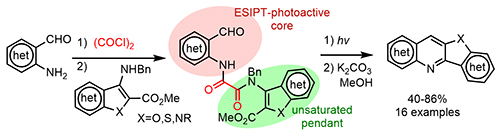
[172]
Kallopterolides A–I, a New Subclass of seco-Diterpenes Isolated from the Southwestern Caribbean Sea Plume Antillogorgia kallos. Marrero, J.; Amador, L.A.; Novitskiy, I.M.; Kutateladze, A.G.; Rodriguiez, A.D. Molecules, 2024, 29, 2493. [DOI: 10.3390/molecules29112493] 
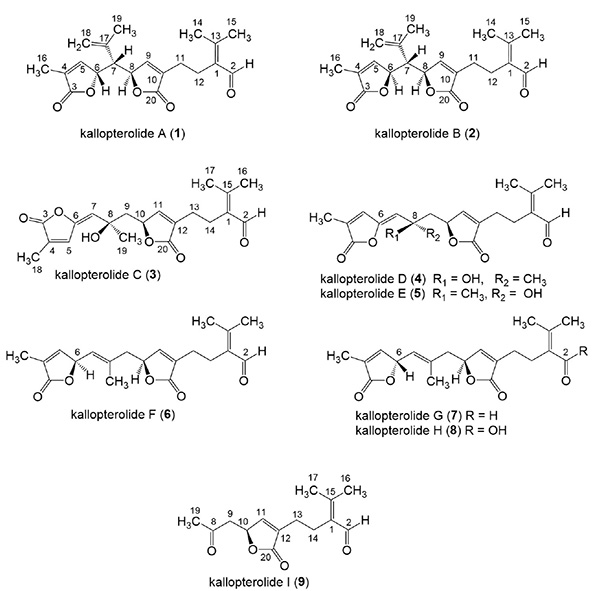
[171]
Complexity-Building Photoinduced Cascade Involving Csp2-Csp3 Coupling of Aromatic Amides via [2 + 2] Reactivity of ESIPT Generated o-Azaxylylenes. Reddy, D.S.; Novitskiy, I.M.; Kutateladze, A.G. Org. Lett., 2023, 25, 1131-1135. [DOI: 10.1021/acs.orglett.3c00092] 
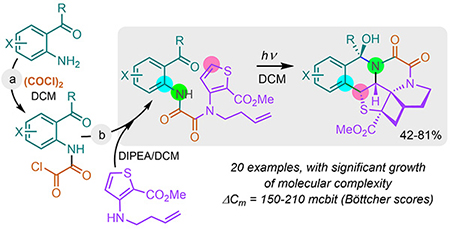
[170]
Complexity-Building ESIPT-Assisted Synthesis of Fused Polyheterocyclic Sulfonamides. Beduru, S.; Kutateladze, A. G. Molecules, 2023, 28, 6549. [DOI: 10.3390/molecules28186549] 
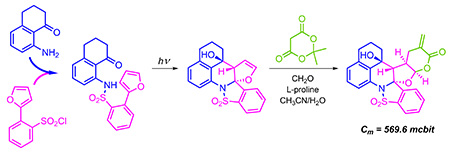
[169]
seco-Pregnane Glycosides from Australian Caustic Vine (Cynanchum viminale subsp. australe) Xue. Y.; Savchenko A.I.; Agnew-Francis, K.A.; Miles, J.A.; Holt, T.; Lu, H.; Chow, S.; Forster, P.I.; Boyle, G. M.; Ross, B.P.; Fisher, K.; Kutateladze, A.G.; Williams, C.M. J. Nat. Prod. , 2023, 86, 490-497. [DOI: 10.1021/acs.jnatprod.2c01037] 
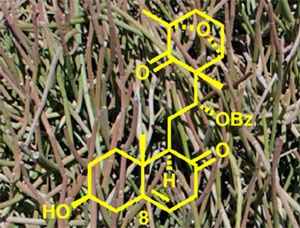
[168]
Arneroma B: Structure reassignment and total synthesis. Deepak, N.M.S.; Kutateladze, A.G.; Elyashberg, M.; Williams, C.M.; Bates, R.W. Tetrahedron, 2023, 147, 133670. [DOI: 10.1016/j.tet.2023.133670] 
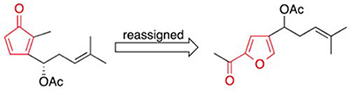
[167]
Structural Reassignment of Two Polyenol Natural Products. Kutateladze, A.G.; Bates, R.W.; Elyashberg, M.; Williams C.M. Eur. J. Org. Chem., 2023, e202201316. [DOI: 10.1002/ejoc.202201316] 
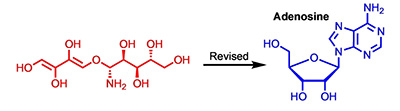
[166]
Reassignmentof the Structureof Janthinolide A. Bates, R.W.; Elyashberg, M.; Kutateladze, A.G.; Williams, C.M. Eur. J. Org. Chem., 2023, 26, e202300910. [DOI: https://doi.org/10.1002/ejoc.202300910] 
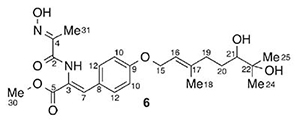
[165]
Rearrangements of the Radical Cations of 3-Aryl-5-fluoroisoxazoles: Further Evidence for Existence of Benzocyclopropenyl Cation. Bondarenko, O.B.; Komarov, A.I.; Nikolaeva, S.N.; Holt, T.A.; Kutateladze, A.G.; Gloriozov, I.P. J. Am. Soc. Mass Spectrom., 2023, 34, 2547-2555. [DOI: 10.1021/jasms.3c00267] 
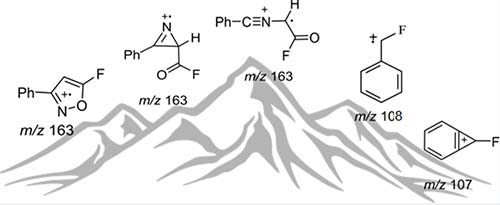
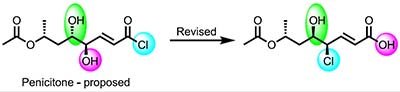
[164]
Penicitone: Structural Reassignment of a Proposed Natural Product Acid Chloride. Novitskiy, I.M.; Elyashberg, M.; Bates, R.W.; Kutateladze, A.G.; Williams, C.M. Org. Lett., 2023, 25, 7796-7799. [DOI: 10.1021/acs.orglett.3c02859] 
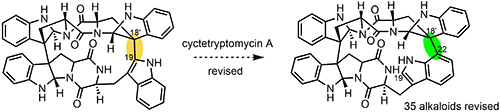
[163]
Brief overview of recently reported misassigned natural products and their in silico revisions enabled by DU8ML, a machine learning-augmented DFT computational NMR method. Novitskiy, I.M.; Kutateladze, A.G. Nat. Prod. Rep., 2022, 39, 2003-2007. [DOI: 10.1039/D2NP00051B] 
[162]
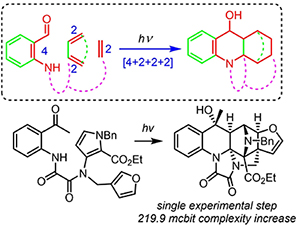
Maximizing Step-Normalized Increases in Molecular Complexity: Formal [4+2+2+2] Photoinduced Cyclization Cascade to Access Polyheterocycles Possessing Privileged Substructures. Reddy, D.S.; Novitskiy, I.M.; Kutateladze, A.G. Angew. Chem. Int. Ed., 2022, 61, (4), e202112573. [DOI: 10.1002/anie.202112573]  Angewandte's Hot Paper
Angewandte's Hot Paper
[161]
Peculiar Reaction Products and Mechanisms Revisited with Machine Learning-Augmented Computational NMR. Novitskiy, I.M.; Kutateladze, A.G. J. Org. Chem., 2022, 87, (13), 8589-8598. [DOI: 10.1021/acs.joc.2c00749]  ACS Editors' Choice
ACS Editors' Choice
[160]
DU8ML: Machine Learning-Augmented Density Functional Theory Nuclear Magnetic Resonance Computations for High-Throughput In Silico Solution Structure Validation and Revision of Complex Alkaloids. Novitskiy, I.M.; Kutateladze, A.G. J. Org. Chem., 2022, 87, (7), 4818-4828. [DOI: 10.1021/acs.joc.2c00169] 
[159]
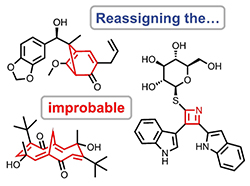
Reassignment of Improbable Natural Products Identified through Chemical Principle Screening. Elyashberg, M.; Novitskiy, I. M.; Bates, R. W.; Kutateladze, A. G.; Williams, C. M. Eur. J. Org. Chem., 2022, 2022, (34), e202200572. [DOI: 10.1002/ejoc.202200572]  (Very Important Paper)
(Very Important Paper)
[158]
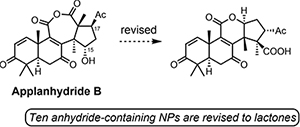
DU8+ Computations Reveal a Common Challenge in the Structure Assignment of Natural Products Containing a Carboxylic Anhydride Moiety. Novitskiy, I.M.; Kutateladze, A.G. J. Org. Chem., 2021, 86, 17511-17515. [DOI: 10.1021/acs.joc.1c02291] 
[157]
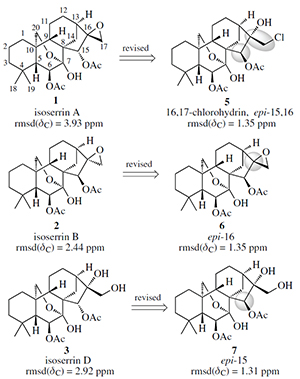
Structure revision of ent-kaurane diterpenoids, isoserrins A, B, and D, enabled by DU8+ computation of their NMR spectral data. Novitskiy, I.M.; Holt, T.A.; Kutateladze, A.G. Mendeleev Comm. , 2021, 31, 300-301. [DOI: 10.1016/j.mencom.2021.05.007] 
[156]
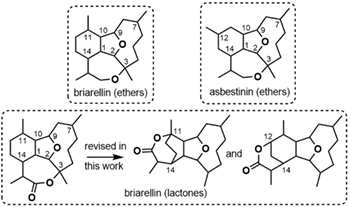
The Discreet Structural Diversity of Briarellins: DU8+ Guided Multiple Structure Revisions Yielded Two Unknown Structural Types. Holt, T. A.; Reddy, D. S.; Huple, D. B.; West, L. M.; Rodriguez, A. D.; Crimmins, M. T.; Kutateladze, A. G. J. Org. Chem., 2020, 85, (9), 6201-6205. [DOI: 10.1021/acs.joc.0c00555] 
[155]
EBC-232 and 323:A Structural Conundrum Necessitating Unification of Five In Silico Prediction and Elucidation Methods. Maslovskaya, L. A.; Savchenk, A. I.; Krenske, E. H.; Chow, S.; Holt, T.; Gordon, V. A.; Reddell, P. W.; Pierce, C. J.; Parsons, P. G.; Boyle, G. M.; Kutateladze, A. G.; Williams, C. M. Chem. Eur. J., 2020, 26, 11862-11867. [DOI: 10.1002/chem.202001884] 
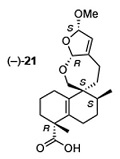
[154]
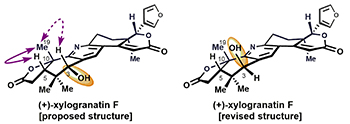
Stereochemical revision of xylogranatin F by GIAO and DU8+ NMR calculations. Liu, Y.; Holt, T. A.; Kutateladze, A. G.; Newhouse, T. R. Chirality, 2020, 32, 515-523. [DOI: 10.1002/chir.23189] 
[153]
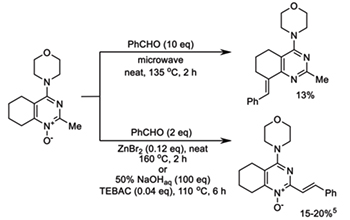
Dichotomy in the reactivity of 2-methyltetrahydroquinazoline 1-oxides towards aldehydes: An unprecedented condensation with simultaneous reduction of the N-oxide fragment. Sedenkova, K. N.;Terekhin, A. V.; Abdrashitova, I. V.; Vasilenko, D. A.; Sadovnikov, K. S.; Gracheva, Y. A.; Grishin, Y. K.; Holt, T. A.; Kutateladze, A. G.; Kuznetsova, T. S.; Milaeva, E. R.; Averina, E. B. Tetrahedron Lett., 2020, 61, (11), 151605. [DOI: 10.1016/j.tetlet.2020.151605] 
[152]
Photoinitiated Cascade for Rapid Access to Pyrroloquinazolinone Core of Vasicinone, Luotonins, and Related Alkaloids. Reddy, D. S.; Kutateladze, A. G. Org. Lett., 2019, 21, (8), 2855-2858.. [DOI: 10.1021/acs.orglett.9b00858] 
[151]
Natural Products Containing the Oxetane and Related Moieties Present Additional Challenge for Structure Elucidation: a DU8+ Computational Case Study. Kutateladze, A. G.; Holt, T. A.; Reddy, D. S. J. Org. Chem., 2019, 84, (12), 7575-7586. [DOI: 10.1021/acs.joc.9b01005]  Featured Article
Featured Article
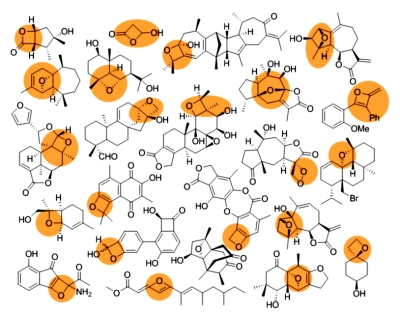
[150]
Structure Validation of Complex Natural Products: Time to Change the Paradigm. What did Synthesis of Alstofolinine A Prove? Kutateladze, A. G.; Holt, T. A. J. Org. Chem., 2019, 84, (12), 8297-8299. [DOI: 10.1021/acs.joc.9b00969] 
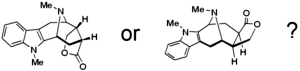
[149]
Diastereoselective heterocyclization of geminal bromo-fluoro arylcyclopropanes by nitrosonium tetrafluoroborate: Access to 4-fluorinated isoxazolines and isoxazoles. Bondarenko, O. B.; Komarov, A. I.; Karetnikov, G. L.; Nikolaeva, S. N.; Zyk, N. V.; Holt, T. A.; Kutateladze, A. G. Tetrahedron, 2019, 75, (46), 130666. [DOI: 10.1016/j.tet.2019.130666] 
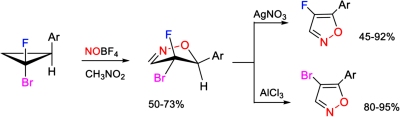
[148]
Reassignments and Corroborations of Oxo-Bridged Natural Products Directed by OSE and DU8+ NMR Computation. Kutateladze, A.G.; Krenske, E.H.; Williams, C.M. Angew. Chem. Int. Ed., 2019, 58, (21), 7107-7112. [DOI: 10.1002/anie.201902777] 
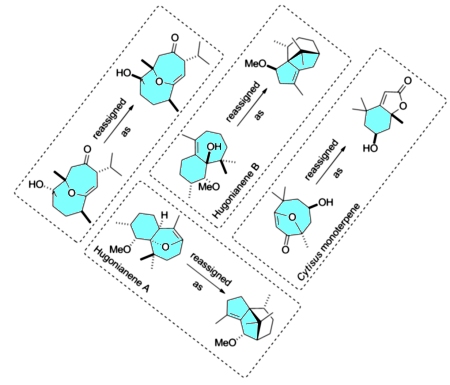
[147]
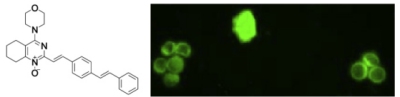
The value of universally available raw NMR data fortransparency, reproducibility, and integrity innatural product research. McAlpine, J.B.; Chen, S.-N.; Kutateladze, A.G.; et. al. Nat. Prod. Rep., 2019, 36, 35-107. [DOI: 10.1039/C7NP00064B] 
[146]
Access to 5-Fluoroisoxazoles via the Nitrosation of Geminal Bromo-Fluoro Arylcyclopropanes. Bondarenko, O.B.; Vinogradov, A.A.; Komarov, A.I.; Karetnikov, G.L.; Zyk, N.V.; Holt, T.; Kutateladze, A.G. Tetrahedron, 2019, 75, 2861-2865. [DOI: 10.1016/j.tet.2019.03.054] 

[145]
Novel π-Conjugated Systems Based on Pyrimidine N-Oxide. Sedenkova, K.N.; Kolodyazhnaya, J.V.; Vasilenko, D.A.; Gracheva Y.A.; Kharitonoshvili, E.V.; Grishin, Y.K.; Chistov, A.A.; Rybakov, V.B.; Holt, T.; Kutateladze, A.G.; Kuznetsova, T.S.; Milaeva, E.R.; Averina, E.B. Dyes and Pigments, 2019, 164, 72-81. [DOI: 10.1016/j.dyepig.2018.12.067] 
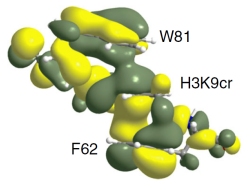
[144]
Structural insights into the π-π-π stacking mechanisms and DNA-binding activity of the YEATS domain. Klein, B.J.; Vann, K.R.; Andrews, F.H.; Wang, W.W.; Zhang, J.; Zhang, Y.; Beloglazkina, A.A.; Mi, W.; Li, Y.; Li, H.; Shi, X.; Kutateladze, A.G.; Strahl, B.D.; Liu, W.R.; Kutateladze, T.G. Nat. Commun., 2018, 9, 4574. [DOI: 10.1038/s41467-018-07072-6] 
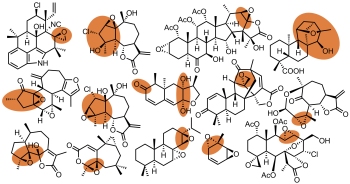
[143]
Addressing the Challenges of Structure Elucidation in Natural Products Possessing the Oxirane Moiety. Kutateladze, A.G.; Kuznetsov, D.M.; Beloglazkina, A. A.; Holt, T. J. Org. Chem., 2018, 83, (15), 8341-8352. [DOI: 10.1021/acs.joc.8b01027]  Featured Article. ACS Editors' Choice (made 20 most read JOC articles in August 2018)
Featured Article. ACS Editors' Choice (made 20 most read JOC articles in August 2018)
[142]
Step-Economical Photoassisted Diversity-Oriented Synthesis: Sustaining Cascade Photoreactions in Oxalyl Anilides to Access Complex Polyheterocyclic Molecular Architectures. Kuznetsov, D.M.; Kutateladze, A.G. J. Am. Chem. Soc., 2017, 139, (46), 16584-16590. [DOI: 10.1021/jacs.7b07598] 
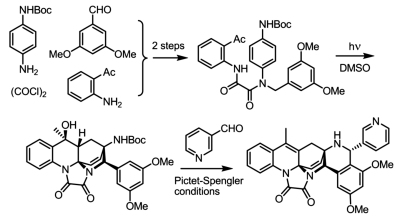
[141]
Triquinanes and Related Sesquiterpenes Revisited Computationally: Structure Corrections of Hirsutanols B and D, Hirsutenol E, Cucumin B, Antrodins C−E, Chondroterpenes A and H, Chondrosterins C and E, Dichrocephone A, and Pethybrene. Kutateladze, A.G.; Kuznetsov, D.M. J. Org. Chem., 2017, 82, (20), 10795-10802. [DOI: 10.1021/acs.joc.7b02018]  Featured Article. ACS Editors' Choice.
Featured Article. ACS Editors' Choice.
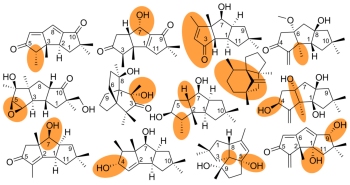
[140]
Structure Determination and Mechanism of Formation of a seco-Moreliane Derivative Supported by Computational Analysis. Chacon Morales, P.A.; Amaro-Luis, J.M.; Kutateladze, A.G. J. Nat. Prod., 2017, 80, (4), 1210-1214. [DOI: 10.1021/acs.jnatprod.7b00041] 
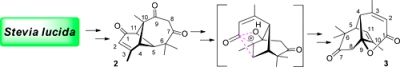
[139]
High-Throughput in Silico Structure Validation and Revision of Halogenated Natural Products Is Enabled by Parametric Corrections to DFT-Computed 13C NMR Chemical Shifts and Spin-Spin Coupling Constants. Kutateladze, A.G.; Reddy, D.S. J. Org. Chem., 2017, 82, (7), 3368-3381. [DOI: 10.1021/acs.joc.7b00188] 
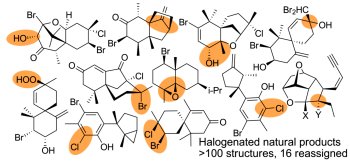
[138]
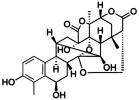
A novel withanolide with an unprecedented carbon skeleton from Physalis angulata. Sun, C.-P.; Kutateladze, A.G.; Zhao, F.; Chen, L.-X.; Qiu, F. Org. Biomol. Chem., 2017, 15, 1110-1114. [DOI: 10.1039/c6ob02656g] 
[137]
Polyheterocycle-carbohydrate chimeras: photoassisted synthesis of 2,5-epoxybenzoxacines and 2,5-epoxybenzazocine scaffolds and their postphotochemical hydroxylations. Reddy, D.S.; Mukhina, O.A.; Cronk, W.C.; Kutateladze, A.G. Pure Applied Chem., 2017, 89, (2), 259-268. [DOI: 10.1515/pac-2016-0915] 
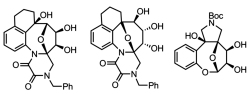
[136]
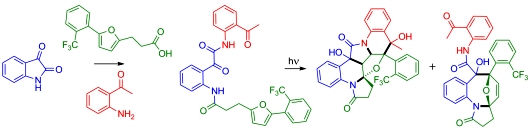
Photoinduced "Double Click" Cascade Offers Access to Complex Polyheterocycles from Readily Available Isatin-Based Photoprecursors. Cronk, W.C.; Mukhina, O.A.; Kutateladze, A.G. Org. Lett., 2016, 18, (15), 3750-3753. [DOI: 10.1021/acs.orglett.6b01775] 
[135]
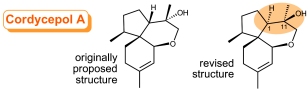
Structure Revision of an Acorane Sesquiterpene Cordycepol A. Reddy, D.S.; Kutateladze, A.G. Org. Lett., 2016, 18, (19), 4860-4863. [DOI: 10.1021/acs.orglett.6b02341] 
[134]
Photoassisted Synthesis of Complex Molecular Architectures: Dearomatization of Benzenoid Arenes with Aza-o-xylylenes via an Unprecedented [2+4] Reaction Topology. Kuznetsov, D.M.; Mukhina, O.A.; Kutateladze, A.G. Angew. Chem., 2016, 55, (24), 6988-6991. [DOI: 10.1002/anie.201602288] 
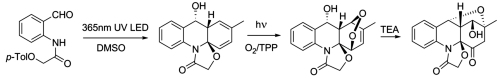
[133]
Structure Revision of Decurrensides A-E Enabled by the RFF Parametric Calculations of Proton Spin-Spin Coupling Constants. Kutateladze, A.G. J. Org. Chem., 2016, 81, (18), 8659-8661. [DOI: 10.1021/acs.joc.6b01855] 
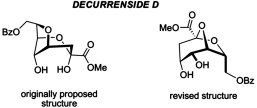
[132]
Oxazolines as Dual Function Traceless Chromophores and Chiral Auxiliaries: Enantioselective Photoassisted Synthesis of Polyheterocyclic Ketones. Mukhina, O.A.; Kutateladze, A.G. J. Am. Chem. Soc., 2016, 138, (7), 2110-2113. [DOI: 10.1021/jacs.5b12690] 
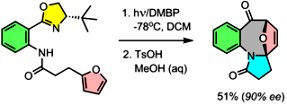
[131]
Computational structure revision of a longipinane derivative meridane. Reddy, D.S.; Kutateladze, A.G. Tetrahedron Lett., 2016, 57, (42), 4727-4729. [DOI: 10.1016/j.tetlet.2016.09.030] 
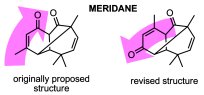
[130]
Photoassisted access to Complex Polyheterocycles Containing a β-lactam moiety. Umstead, W.J.; Mukhina, O.A.; Kutateladze, A.G. J. Photochem. Photobiol. A: Chem., 2016, 329, 182-188. [DOI: 10.1016/j.jphotochem.2016.07.004] 
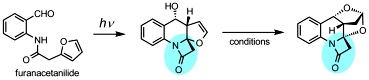
[129]
Beyond the Dimer and Trimer: Tetraspiro[2.1.25.1.29.1.213.13]hexadecane-1,3,5,7-tetraone - the Cyclic Tetramer of Carbonylcyclopropane. Sedenkova, K.N.; Averina, E.B.; Grishin, Y.K.; Andriasov, K.S.; Stepanova, S.A.; Roznyatovsky, V. A.; Kutateladze, A.G.; Rybakov, V.B.; Albov, D.V.; Kuznetsova, T.S.; Zefirov, N.S. Chem. Eur. J., 2016, 22, (12), 3996-3999. [DOI: 10.1002/chem.201600140] 
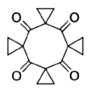
[128]
Photoassisted Diversity-Oriented Synthesis: Intramolecular Cycloadditions of Photogenerated Azaxylylenes with Oxazole Pendants, and Subsequent Postphotochemical Multicomponent Modifications. Kumar, N.N.B.; Kutateladze, A.G. Org. Lett., 2016, 18, (3), 460-463. [DOI: 10.1021/acs.orglett.5b03503] 
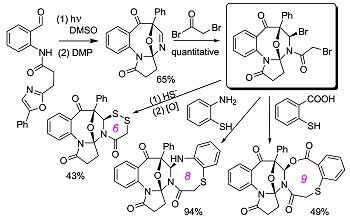
[127]
Amino Azaxylylenes Photogenerated from o-Amido Imines: Photoassisted Access to Complex Spiro-Polyheterocycles. Mukhina, O.A.; Kuznetsov, D.M.; Cowger, T.M.; Kutateladze, A.G. Angew. Chem. Int. Ed., 2015, 54, (39), 11516-11520. [DOI: 10.1002/anie.201504455] 
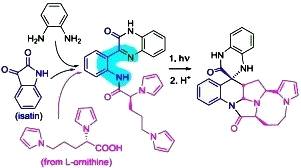
[126]
Relativistic Force Field: Parametrization of 13C-1H Nuclear Spin-Spin Coupling Constants. Kutateladze, A.G.; Mukhina, O.A. J. Org. Chem., 2015, 80, (21), 10838-10848. [DOI: 10.1021/acs.joc.5b02001] 

[125]
Photoinduced Cycloadditions in the Diversity-Oriented Synthesis Toolbox: Increasing Complexity with Straightforward Postphotochemical Modifications. Umstead, W.J.; Mukhina, O.A.; Kumar, N.N.B.; Kutateladze, A.G. Aust. J. Chem., 2015, 68, 1672-1681. [DOI: 10.1071/CH15266] 
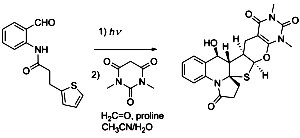
[124]
Computationally driven reassignment of the structures of aldingenins A and B. Mukhina O.A.; Koshino, H.; Crimmins M.T.; Kutateladze, A.G. Tetrahedron Lett. , 2015, 56, 4900-4903. [DOI: 10.1016/j.tetlet.2015.06.078] 
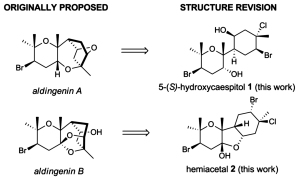
[123]
Minimalist Relativistic Force Field: Prediction of Proton-Proton Coupling Constants in 1H NMR Spectra is Perfected with NBO Hybridization Parameters. Kutateladze, A.G.; Mukhina, O.A. J. Org. Chem., 2015, 80, (10), 5218-5225. [DOI: 10.1021/acs.joc.5b00619] 
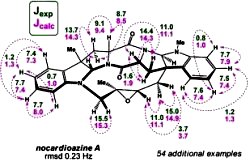
[122]
Intramolecular Cycloadditions of Photogenerated Azaxylylenes with Oxadiazoles Provide Direct Access to Versatile Polyheterocyclic Ketopiperazines Containing a Spiro-Oxirane Moiety. Kumar, N.N.B.; Kuznetsov, D.M.; Kutateladze, A.G. Org. Lett. , 2015, 17, (3), 438-441. [DOI: 10.1021/ol5033909] 
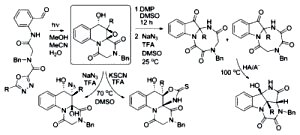
[121]
Conformationally Constrained Penta(hetero)cyclic Molecular Architectures via Photoassisted Diversity-Oriented Synthesis. Umstead, W.J.; Mukhina, O.A.; Kutateladze, A.G. Eur. J. Org. Chem., 2015, (10), 2205-2213. [DOI: 10.1002/ejoc.201403620] 
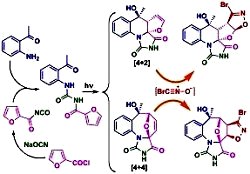
[120]
An acetyl-methyl switch drives a conformational change in p53. Tong, Q.; Mazur, S.J.; Rincon-Arano, H.; Rothbart, S.; Kuznetsov, D.M.; Cui, G.; Liu, W.H.; Gete, Y.; Klein, B.J.; Jenkins, L.; Mer, G.; Kutateladze, A.G.; Strahl, B.D.; Groudine, M.; Appella, E.; Kutateladze, T.G. Structure, 2015, 23, (2), 322-331. [DOI: 10.1016/j.str.2014.12.010] 
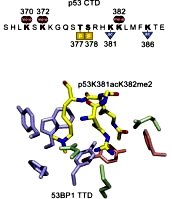
[119]
Photoassisted Diversity-Oriented Synthesis: Accessing 2,6-Epoxyazocane (Oxamorphan) Cores. Mukhina, O.A.; Kumar, N.N.B.; Cowger, T.M.; Kutateladze, A.G. J. Org. Chem., 2014, 79, (22), 10956-10971. [DOI: 10.1021/jo5019848] 
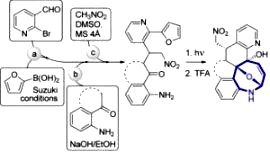
[118]
Relativistic Force Field: Parametric Computations of Proton-Proton Coupling Constants in 1H NMR Spectra. Kutateladze, A.G.; Mukhina, O.A. J. Org. Chem., 2014, 79, (17), 8397-8406. [DOI: 10.1021/jo501781b] 
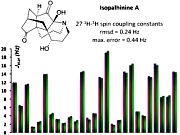
[117]
Intramolecular Cycloadditions of Photogenerated Azaxylylenes: an Experimental and Theoretical Study. Mukhina, O.A.; Cronk, W.C.; Kumar, N.N.B.; Sekhar, M.; Samanta, A.; Kutateladze, A.G. J. Phys. Chem. A., 2014, 118, (45), 10487-10496. [DOI: 10.1021/jp504281y] 
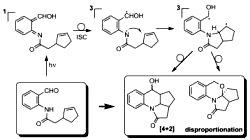
[116]
symm-Tetramethylenecyclooctane: En Route to Polyspirocycles. Averina, E.B.; Sedenkova, K.N.; Bakhtin, S.G.; Grishin, Y.K.; Kutateladze, A.G.; Roznyatovsky, V.A.; Rybakov, V.B.; Butov G.M.; Kuznetsova, T.S.; Zerfirov, N.S. J. Org Chem., 2014, 79, (17), 8163-8170. [DOI: 10.1021/jo501380y] 
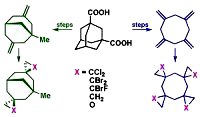
[115]
Photoactive Spatial Proximity Probes for Binding Pairs with Epigenetic Marks. Ezhov, R.N.; Metzel, G.A.; Mukhina, O.A.; Musselman, C.A.; Kutateladze, T.G; Gustafson, T.P.; Kutateladze, A.G. J. Photochem. Photobiol. A: Chemistry, 2014, 290, 101-108. [DOI: 10.1016/j.jphotochem.2014.06.014] 
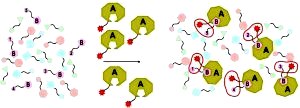
[114]
Intramolecular Photoassisted Cycloadditions of Azaxylylenes and Post-Photochemical Capstone Modifications via Suzuki Coupling Provide Access to Complex Polyheterocyclic Biaryls. Cronk, W.C.; Mukhina, O.A.; Kutateladze, A.G. J. Org. Chem., 2014, 79, (3), 1235-1246. [DOI: 10.1021/jo4026447] 
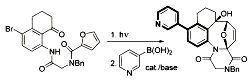
[113]
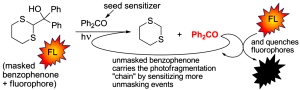
Photoinduced signal amplification through externally sensitized photofragmentation in masked photosensitizers. Kutateladze, A. G.; Kurchan, A. N.; Kottani, R.; Majjigapu, J. R. R. US 8,735,167 B2, 2014, May27. 
[112]
Photoassisted Synthesis of Enantiopure Alkaloid Mimics Possessing Unprecedented Polyheterocyclic Cores. Kumar, N.N.B.; Mukhina, O.A.; Kutateladze, A.G. J. Am. Chem. Soc., 2013, 135, (26), 9608-9611. [DOI: 10.1021/ja4042109] 
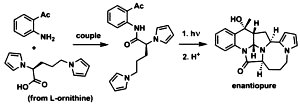
[111]
Photoassisted Access to Enantiopure Conformationally Locked Ribofuranosylamines Spiro-Linked to Oxazolidino- Diketopiperazines. Nandurkar, N.S.; Kumar, N.N.B.; Mukhina, O.A.; Kutateladze, A.G. ACS Combinatorial Sci., 2013, 15, (1), 73-76. [DOI: 10.1021/co3001296] 
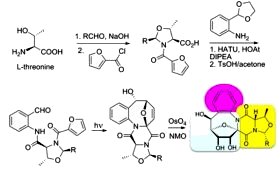
[110]
Photoinduced Intramolecular Cyclopentanation vs Photoprotolytic Oxametathesis in Policyclic Alkenes Outfitted with Conformationally Constrained Aroylmethyl Chromophores. Valiulin, R.A.; Arisco, T.M.; Kutateladze, A.G. J. Org. Chem., 2013, 78, (5), 2012-2025. [DOI: 10.1021/jo301909j] 
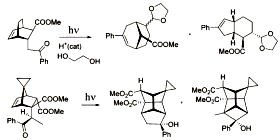
[109]
Cascade Transformations Involving Thiocarbonyls: Photoassisted Access to Bicyclic Thiiranes and Oxapentalenes. Valiulin, R.A.; Kumar, N.N.B.; Kuznetsov, D.M.; Kutateladze, A.G. J. Sulfur Chem., 2013, 34, (1-2), 209-221. [DOI: 10.1080/17415993.2012.731065] 
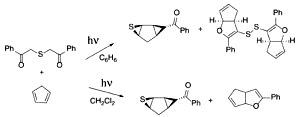
[108]
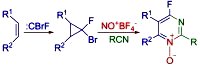
Three-Component Heterocyclization of gem-Bromofluorocyclopropanes with NOBF4: Access to 4-Fluoropyrimidine N-Oxides Sedenkova, K.N.; Averina, E.B.; Grishin, Y.K.; Kutateladze, A.G.; Rybakov, V.B.; Kuznetsova, T.S.; Zefirov, N.S. J. Org. Chem., 2012, 77, (21), 9893-9899. [DOI: 10.1021/jo301880m] 
[107]
Externally Sensitized Deprotection of PPG-Masked Carbonyls as a Spatial Proximity Probe in Photoamplified Detection of Binding Events. Gustafson, T.P.; Metzel, G.A.; Kutateladze, A.G. Photochem. Photobiol. Sci., 2012, 11, (3), 564-577. [DOI: 10.1039/C2PP05326H] 

[106]
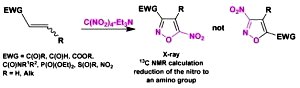
Heterocyclization of Electrophilic Alkenes with Tetranitromethane Revisited: Regiochemistry and the Mechanism of Nitroisoxazoles Formation. Averina, E.B.; Samoilichenko, Y.V.; Volkova, Y.A.; Grishin, Y.K.; Rybakov, V.B.; Kutateladze, A.G.; Elyashberg, M.E.; Kuznetsova, T.S.; Zefirov, N.S. Tetrahedron Lett., 2012, 53, (12), 1472-1475. [DOI: 10.1016/j.tetlet.2012.01.039] 
[105]
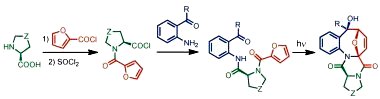
Rapid Photoassisted Access to N,O,S-Polyheterocycles with Benzoazocine and Hydroquinoline Cores via Intramolecular Cycloadditions of Photogenerated Azaxylylenes Mukhina, O.A.; Kumar, N.N.B.; Arisco, T.M.; Valiulin, R.A.; Metzel, G.A.; Kutateladze, A.G. Angew. Chem. Int. Ed. , 2011, 50, (40), 9423-9428. [DOI: 10.1002/anie.201103597]  Angewandte's Hot Paper (click here)
Angewandte's Hot Paper (click here)
[104]
Photochemically Amplified Detection of Molecular Recognition Events: An Ultra-Sensitive Fluorescence Turn-Off Binding Assay. Gustafson, T.P.; Metzel, G.A.; Kutateladze, A.G. Org. Biomol. Chem., 2011, 9, (13), 4752-4755. [DOI: 10.1039/C1OB05289F]  Selected as an OBC HOT Article (click here)
Selected as an OBC HOT Article (click here)
[103]
Double-tandem [4π+2π]-[2π+2π]-[4π+2π]-[2π+2π] Synthetic Sequence with Photoprotolytic Oxametathesis and Photoepoxidation in the Chromone Series. Valiulin, R.A.; Arisco, T.M., Kutateladze, A.G. J. Org. Chem., 2011, 76, (5), 1319-1332. [DOI: 10.1021/jo102221q] 
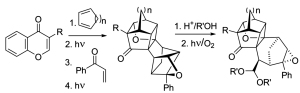
[102]
Strained to the Limit: When a Cyclobutyl Moiety Becomes a Thermodynamic Sink in a Protolytic Ring Opening of Photogenerated Oxetanes Valiulin, R.A.; Arisco, T.M., Kutateladze, A.G. Org. Lett., 2010, 12, (15), 3398-3401. [DOI: 10.1021/ol101297b] 
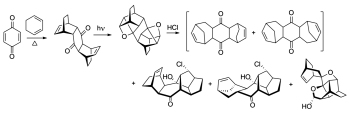
[101]
First Example of Intramolecular [2π+2π] Alkene-Arene Photocyclization in the Chromone Series and its Synthetic Utility Valiulin, R.A.; Kutateladze, A.G. Tetrahedron Lett., 2010, 51, (29), 3803-3806. [DOI: 10.1016/j.tetlet.2010.05.078] 
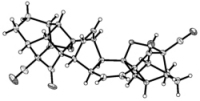
[100]
Harvesting the Strain Installed by a Patern?B?hi Step in a Synthetically Useful Way: High Yielding Photo-Protolytic Oxametathesis in Polycyclic Systems. Valiulin, R.A.; Kutateladze, A.G. Org. Lett., 2009, 11, (17), 3886-3889. [DOI: 10.1021/ol901456m] 
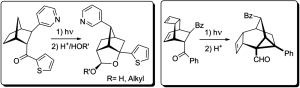
[99]
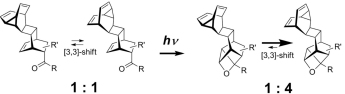
Effect of Intramolecular Patern?B?hi Reaction on the Thermodynamics and Kinetics of Nearly Degenerate [3,3]-Sigmatropic Shift in Fluxional Polycycles. Valiulin, R.A.; Dressen, D.G.; Riggs, J.R.; Habte, F.M.; Kutateladze, A.G. J. Org. Chem., 2009, 74, (9), 3484-3490. [DOI: 10.1021/jo9003822] 
[98]
Interaction of Radical Pairs Through-Bond and Through-Space: Scope and Limitations of the Point-Dipole Approximation in Electron Paramagnetic Resonance Spectroscopy. Riplinger, C.; Kao, J.; Rosen, G.; Kathirvelu, V.; Eaton, G.; Eaton, S.; Kutateladze, A.; Neese, F. J. Am. Chem. Soc., 2009, 131, (29), 10092-10106. [DOI: 10.1021/ja901150j] 
[97]
A Peculiar Quenching Concentration Dependence of Photoinduced Fragmentation in Dithiane-Carbonyl Adducts: A Mechanistic Experimental and Theoretical Study. Valiulin, R.A.; Lakkakula, S.; Kutateladze, A.G. J. Photochem. Photobiol. A Chemistry, 2009, 206, 80-86. [DOI: 10.1016/j.jphotochem.2009.05.016] 
[96]
Membrane insertion of the FYVE domain is modulated by pH. He, J.; Vora, M.; Haney, R.M.; Filonov, G.S.; Musselman, C.A.; Burd, C.G.; Kutateladze, A.G.; Verkhusha, V.V.; Stahelin, R.A.; Kutateladze, T.G. Proteins, 2009, 76, (4), 852-860. [DOI: 10.1002/prot.22392] 
[95]
Effect of beta-Alkylthioethyl Substitution in 1,3-Dithianes: Quasi-Anchimeric Assistance in Photoinduced Electron Transfer? Valiulin, R.A.; Kutateladze, A.G. J. Org. Chem., 2008, 73, (16), 6393-6396. [DOI: 10.1021/jo800938d] 
[94]
Photolabile Amphiphiles with Fluorogenic Thioxanthone-Dithiane Functionality: Synthesis and Photoinduced Fragmentation in Micelles. Ezhov, R.N.; Rozhkov, V.V.; Majjigapu, J.R.R.; Kutateladze, A.G. J. Sulf. Chem., 2008, 29, (3-4), 389-400. [DOI: 10.1080/17415990802027289] 
[93]
Protocols on safety, efficacy, standardization, and documentation of herbal medicine. Mosihuzzaman, M.; et al. Pure Appl. Chem. , 2008, 80, (10), 2195-2230. 
[92]
2,6,7-Trithiabicyclo[2.2.2]octanes as Promising Photolabile Tags for Combinatorial Encoding. Valiulin, R.A.; Kutateladze, A.G. J. Org. Chem., 2008, 73, (1), 335-338. [DOI: 10.1021/jo702091e] 
[91]
Photoamplification and Multiple Tag Release in a Linear Peptide-Based Array of Dithiane Adducts Majjigapu, K.; Majjigapu, J.R.R.; Kutateladze, A.G. Angew. Chem., 2007, 46, (32), 6137-6140. [DOI: 10.1002/anie.200701512] 
[90]
Interrupted Oligomerization Revisited: Simple and Efficient One Pot Multicomponent Approach to Versatile Synthetic Intermediates. Valiulin, R.A.; Halliburton, L.M.; Kutateladze, A.G. Org. Lett., 2007, 9, (20), 4061-4063. [DOI: 10.1021/ol701847b] 
[89]
Method for Encoding and Screening Combinatorial Libraries. Kottani, R.; Valiulin, R. A.; Majjigapu, J. R. R., Kutateladze, A. G. WO2006US61728, 2007, June_21,  esp@cenet
esp@cenet
[88]
Photoactive Barbiturate Receptors: An Ultimate Lock-and-Key System in Which the Key Unlocks the Lock. Lakkakula, S.; Mitkin, O.D.; Valiulin, R.A.; Kutateladze, A.G. Org. Lett., 2007, 9, (6), 1077-1079. [DOI: 10.1021/ol0700153] 
[87]
Bis-(4-(2-pyridylmethyleneiminophenyl)disulfide - a Chelating Ligand Capable of Self Assembly on Gold Surface and Its Complexes with M(BF4)2 and M(ClO4)2; M = Co, Cu and Ni. Experimental and Theoretical Study. Beloglazkina, E. K.; Majouga, A. G.; Zyk, N. V.; Rakhimov, R. D.; Yaminsky, I. V.; Gorelkin, P.; Kiselev, G. A.; Kutateladze, A. G. Thin Solid Films, 2007, 515, (11), 4649-4661. [DOI: 10.1016/j.tsf.2006.12.131] 
[86]
Photolabile system with instantaneous fluorescence reporting function. Kutateladze, A. G.; Kurchan, A. N.; Kottani, R.; Majjigapu, J. R. R. WO2007008471 , 2007, January_18,  esp@cenet
esp@cenet
[85]
Glossary of Terms Used In Photochemistry, 3d Edition Braslavsky S.E. et al. Pure Appl. Chem., 2007, 79, (3), 293-465. [DOI: 10.1351/pac200779030293] 
[84]
Photoinduced Signal Amplification Through Controlled Externally Sensitized Fragmentation in Masked Sensitizers. Kottani, R.R.; Majjigapu, J.R.R.; Kurchan, A.N.; Majjigapu, K.; Gustafson, T.P.; Kutateladze, A.G. J. Am. Chem. Soc., 2006, 128, (46), 14794-14795. [DOI: 10.1021/ja066692u] 
[83]
Direct Screening of Solution Phase Combinatorial Libraries Encoded with Externally Sensitized Photolabile Tags. Kottani, R.; Valiulin, R. A.; Kutateladze, A. G. Proc. Natl. Acad. Sci. USA, 2006, 103, (38), 13917-13921. [DOI: 10.1073/pnas.0606380103] 
[82]
Conformational Analysis of Spiro-Bis-Dithiepins: a Peculiar Case of Axial Chirality Wade, E.O.; Valiulin, R.A.; Ruybal, L.A.; Kutateladze, A.G. Org. Lett., 2006, 8, (22), 5121-5124. [DOI: 10.1021/ol062172s] 
[81]
When Ethyl is Infinitely Different from Methyl: Double Addition of Lithiated Dithianes to Aromatic Carboxylates Revisited. Valiulin, R. A.; Kottani, R.; Kutateladze, A. G. J. Org. Chem, 2006, 71, (13), 5047-5049. [DOI: 10.1021/jo060780f] 
[80]
Externally Sensitized Mesolytic Fragmentations in Dithiane-Ketone Adducts. Gustafson, T. P.; Kurchan, A. N.; Kutateladze, A. G. Tetrahedron, 2006, 62, (27), 6574-6580. [DOI: 10.1016/j.tet.2006.03.065] 
[79]
Release and Report: A New Photolabile Caging System with a Two-photon Fluorescence Reporting Function. Majjigapu, J. R. R.; Kurchan, A. N.; Kottani, R.; Gustafson, T. P.; Kutateladze, A. G. J. Am. Chem. Soc., 2005, 127, (36), 12458-12459. [DOI: 10.1021/ja053654m] 
[78]
Investigation of the Binding Geometry of a Peripheral Membrane Protein. Brunecky, R.; Lee, S.; Rzepecki, P.W.; Overduin, M.; Prestwich, G.D.; Kutateladze, A.G.; Kutateladze, T.G. Biochem., 2005, 44, (49), 16064-16071. [DOI: 10.1021/bi051127+] 
[77]
Dithiane and Trithiane-Based Photolabile Molecular Linkers Equipped with Amino-Functionality: Synthesis and Quantum Yields of Fragmentation. Kurchan, A. N.; Mitkin, O. D.; Kutateladze, A. G. J. Photochem. Photobiol. A: Chemistry, 2005, 171, 121-129. [DOI: 10.1016/j.jphotochem.2004.09.014] 
[76]
Computational Methods in Photochemistry. Kutateladze, A. G., Editor; CRC Press, Molecular and Supramolecular Photochemistry, 2005,  Vol 13.
Vol 13.
[75]
Toward Parameterization of Spin-Orbit Coupling in Triplet Organic Diradicals Separated by a Partially Conjugated Spacer. Kutateladze, A.G.; McHale, W.A.Jr. ARKIVOC, 2005, IV, 88-101. 
[74]
Dithiane, Trithiane and Dithiazane-Based Photolabile Scaffolds for Molecular Recognition: Mechanism and Efficiency of the Photoinduced Fragmentation in Aqueous Reductive Environments. Kutateladze, A.G; Kottani, R.; Kurchan, A. N.; Majjigapu, J. R. R.; Shirk, S. M. Phosph. Sulf. Silicon, 2005, 180, (5-6), 1379-1384. 
[73]
Addition of Lithiated 5 Hydroxymethyl-1,3 Dithiane to Benzaldehyde: HMPA-Controlled trans-Stereoselectivity Li, Z.; Kurchan, A. N.; Kutateladze, A. G. Org. Lett., 2004, 6, (8), 1213-1216. [DOI: 10.1021/ol0499497] 
[72]
Dithiane and Trithiane-Based Photocleavable Systems for Molecular Assembly and Disassembly. Kurchan, A. N.; Shirk, S. M.; Kutateladze, A. G. The Spectrum, 2004, 17, (4), 20-25. 
[71]
Multivalent Mechanism of Membrane Insertion by the FYVE Domain. Kutateladze, T. G.; Capelluto, D. G. S.; Ferguson, C. G.; Cheever, M. L.; Kutateladze, A. G.; Prestwich, G. D.; Overduin, M. J. Biol. Chem., 2004, 279, (4), 3050-3057. 
[70]
Anomalous C-C Bond Cleavage in Sulfur-Centered Cation Radicals Containing Vicinal Hydroxy Group. Li, Z.; Kutateladze, A. G. J. Org. Chem., 2003, 68, (21), 8236-8239. [DOI: 10.1021/jo035001z] 
[69]
ICP21 Report. Kutateladze, A. G. IAPS Newsletter, 2003, 26, (1), 11-15. 
[68]
Novel Dithia-aza-norbornanes as "Stiff" Bicyclic Dithiazines. Kurchan, A. N.; Wade, E.; Kutateladze, A. G. Synlett , 2003, (11), 1731-1733. [DOI: 10.1055/s-2003-41431] 
[67]
Dithiane-Based Photolabile Amphiphiles: Toward Photolabile Liposomes. Li, Z.; Wan, Y.; Kutateladze, A. G. Langmuir, 2003, 19, (16), 6381-6391. [DOI: 10.1021/la034188m] 
[66]
Photolabile Calixarene-Based Rosette. Li, Z.; Chiu, H.; Kutateladze, A. G. Can. J. Chem., 2003, 81, (6), 807-810. [DOI: 10.1139/V03-057] 
[65]
Amino Acid-Based Dithiazines: Synthesis and Photofragmentation of Their Benzaldehyde Adducts. Kurchan, A. N.; Kutateladze, A. G. Org. Lett., 2002, 4, (23), 4129-4131. [DOI: 10.1021/ol0268790] 
[64]
Liposomes from Novel Photolabile Phospholipids: Light-Induced Unloading of Small Molecules as Monitored by PFG NMR. Wan, Y.; Angleson, J. K.; Kutateladze, A. G. J. Am. Chem. Soc., 2002, 124, (20), 5610-5611. [DOI: 10.1021/ja016874i] 
[63]
Synthesis and Liquid Membrane Transport Properties of Photolabile Molecular Clips Based on Dithiane-Spiro-Crown Ethers. Barnhurst, L. A.; Kutateladze, A. G. Org. Lett., 2001, 3, (17), 2633-2635. [DOI: 10.1021/ol016309k] 
[62]
Conformational Analysis of Singlet-Triplet State Mixing in Paterno-Buchi Diradicals. Kutateladze, A. G. J. Am. Chem. Soc., 2001, 123, (38), 9279-9282. [DOI: 10.1021/ja016092p] 
[61]
Synthesis of Dithiane-Based Photolabile Molecular Systems. Mitkin, O.; Wan, Y.; Kurchan, A.; Kutateladze, A. Synthesis, 2001, (8), 1133-1142. 
[60]
Photoinduced C-C Bond Cleavage in Dithiane-Carbonyl Adducts: A Laser Flash Photolysis Study. Vath, P.; Falvey, D. E.; Barnhurst, L. A.; Kutateladze, A. G. J. Org. Chem., 2001, 66, (8), 2886-2890. [DOI: 10.1021/jo010102n] 
[59]
Dithiane and Trithiane-Based Photolabile Scaffolds for Molecular Recognition. Mitkin, O. D.; Kurchan, A. N.; Wan, Y.; Schiwal, B. F.; Kutateladze, A. G. Org. Lett., 2001, 3, (12), 1841-1844. [DOI: 10.1021/ol015933u] 
[58]
Photoinduced 1,3-Proton Shift in Methyldithiepines as a Potential Way of Modulating Hyperpolarizabilities. Wan, Y.; Kurchan, A. N.; Kutateladze, A. G. J. Org. Chem., 2001, 66, (5), 1894-1899. [DOI: 10.1021/jo005707i] 
[57]
Determination of the Position of the Conformational Equilibrium of a trans-1,2-Disubstituted Cyclohexane by NMR Spectroscopy. An Experiment in Physical Organic Chemistry for Undergraduate Students. Kutateladze, A. G.; Hornback J. M. J. Chem. Ed., 2001, 78, (1), 81-82. 
[56]
Molecular Assembly and Disassembly: Novel Photolabile Molecular Hosts. Wan, Y.; Mitkin, O.; Barnhurst L.; Kurchan, A.; Kutateladze, A. Org. Lett., 2000, 2, (24), 3817-3819. [DOI: 10.1021/ol006692d] 
[55]
Direct Transformation of 1,3-Dihalides into Dithianes and Dithiepins via a Novel One Pot Reaction with Carbon Disulfide and Sodium Borohydride. Wan, Y.; Kurchan, A. N.; Barnhurst L. A.; Kutateladze, A. G. Org. Lett., 2000, 2, (8), 1133-1135. [DOI: 10.1021/ol005705k] 
[54]
Efficient Electrochemical Deprotection of Carboxylic and Amino Acids from Their 2-(Hydroxymethyl)-1,3-dithiane (Dim) Esters. Barnhurst, L. A.; Wan, Y.; Kutateladze A. G. Org. Lett. , 2000, 2, (6), 799-801. [DOI: 10.1021/ol005537w] 
[53]
Photooxidation of Methyldithiepins into Dithiepin Carboxaldehydes in Carbon Tetrachloride. Wan Y., Barnhurst, L. A.; Kutateladze A. G. Org. Lett. , 1999, 1, (6), 937-939. [DOI: 10.1021/ol990870p] 
[52]
Reactions of Nitrosonium Ethyl Sulfate with Olefins and Dienes: An Experimental and Theoretical Study. Zyk, N. V.; Nesterov, E. E.; Khlobystov, A. N.; Zefirov, N. S.; Barnhurst, L. A.; Kutateladze, A. G. J. Org. Chem. , 1999, 64, (19), 7121-7128. [DOI: 10.1021/jo990679t] 
[51]
An Efficient Photo-SET-Induced Cleavage of Dithiane-Carbonyl Adducts and Its Relevance to the Development of Photoremovable Protecting Groups for Ketones and Aldehydes. McHale, W. A.; Kutateladze A. G. J. Org. Chem. , 1998, 63, (26), 9924-9931. [DOI: 10.1021/jo981697y] 
[50]
Tin-Free Reductive Photochemical Carboxymethylation of Olefins with α-Alkylthioacetates. Deng, L. X.; Kutateladze, A. G. Tetrahedron Lett. , 1997, 38, (44), 7829-7832. 
[49]
New Approaches to Singlet-Triplet Spin-Orbit Coupling in Photochemistry and Diradical Chemistry; Mechanistic and Exploratory Organic Photochemistry. Zimmerman, H. E.; Kutateladze, A.G. J. Am. Chem. Soc. , 1996, 118, (1), 249-250. [DOI: 10.1021/ja953052a] 
[48]
S-Tosylthiosulfenamides as electrophilic sulfosulfenylating reagents. Zyk N.V.; Beloglazkina E.K.; Alabugin I.V.; Kutateladze A.G.; Zefirov N.S. Bulletin of Moscow State University (Vestnik MGU), Ser.Khimia, , 1996, 37, (1), 68-70. 
[47]
Novel Dissection Analysis of Spin-Orbit Coupling in the Type-B Cyclohexenone Photorearrangement. What Controls Photoreactivity? Mechanistic and Exploratory Organic Photochemistry. Zimmerman, H.E.; Kutateladze, A.G. J. Org. Chem. , 1995, 60, (19), 6008. 
[46]
Sulfoxylic Acid Derivatives as Novel Sulfenylating Reagents. Vatsadze, S.Z.; Zefirov, N.S.; Zyk, N.V.; Kutateladze, A.G. Phosphorus, Sulfur, and Silicon , 1994, 95, 333. 
[45]
Excited State Reactivity as a Function of Diradical Structure. Evidence for Two Triplet Cyclopropyl-dicarbinyl Diradical Intermediates With Differing Reactivity. Zimmerman, H.E.; Kutateladze, A.G.; Maekawa, Y.; Mangette, J.E. J. Am. Chem. Soc. , 1994, 116, (21), 9795. 
[44]
Electrophilic Sulfamatoselenenylation of Olefins. Zyk, N.V., Alabugin, I.V.; Kutateladze, A.G.; Kice, J.L.; Zefirov, N.S. Dokl. Acad. Nauk SSSR , 1994, 317, (2), 208. 
[43]
A One-pot Trifunctionalization of Olefins with Benzeneseleninic and Trifluoroacetic Anhydrides Using a Commonly Undesirable Side Reaction as a Key Step. Kutateladze, A.G.; Kice, J.L.; Kutateladze, T.G.; Zefirov, N.S. J. Org. Chem., 1993, 58, 995. 
[42]
Thiosulfonates: synthesis, reactions and practical application. (Review). Zefirov, N.S.; Zyk, N.V.; Beloglazkina, E.K.; Kutateladze, A.G. Sulfur Reports, 1993, 14, 223. 
[41]
Phenylethynesulfenyl sulfamate - a new sulfenylating reagent. Denisko, O.V.; Kutateladze, A.G.; Zyk, N.V.; Zefirov, N.S. Sulfur Lett., 1993, 14, 341. 
[40]
The Markedly Enhanced Basicity of Selenenamides vs. Sulfenamides and the Mechanism of the Methanolysis of o-Nitro- and 2,4,6-Tri-tert-butylbenzeneselenenamides. Kice, J.L; Kutateladze, A.G. J. Org. Chem., 1993, 58, 917. 
[39]
S-Tosylsulfenyl Chloride - the First Representative of a New Class of Sulfur Halides. Kutateladze, A.G.; Beloglazkina, E.K.; Zyk, N.V.; Zefirov, N.S. Izv. Akad. Nauk SSSR, Ser. Khim. , 1992, (5), 1217. 
[38]
Sulfato-sulfenylation of Olefins by Ethyl Phenylsulfenate in the Presence of Sulfur Trioxide. Zefirov, N.S.; Zyk, N.V.; Lapin, Yu.A.; Kutateladze, A.G.; Ugrak, B.I. Zh. Org. Chem. , 1992, 28, (6), 1126. 
[37]
Superelectrophilic Selenium. A New Simple Method for Generation of Areneselenenyl Trifluoroacetates and Triflates. Kutateladze, A.G.; Kice, J.L.; Kutateladze, T.G.; Zefirov, N.S.; Zyk, N.V. Tetrahedron Lett., 1992, 33, 1949. 
[36]
Reactions of sulfenic and sulfoxylic acid derivatives with olefins in the presence of sulfur trioxide and its complexes. (Review). Kutateladze, A.G.; Zefirov, N.S.; Zyk, N.V. Sulfur Reports, 1992, 11, (2), 233.  [CA: 117, 130842w]
[CA: 117, 130842w]
[35]
Mechanism of Nucleophilic Substitution Reactions of o-Nitrobenzene-sulfenamides: Evidence for a Substitution Proceeding through a Sulfuranide Intermediate. Kice, J.L.; Kutateladze, A.G. J. Org. Chem., 1992, 57, 3298. 
[34]
A Simple New Synthesis of Thiobisamines. Kutateladze, T.G.; Kice, J.L.; Kutateladze, A.G.; Zefirov, N.S.; Zyk, N.V. J. Org. Chem. , 1991, 56, 5235. 
[33]
S-Tosylsulfenamides as new electrophilic reagents for sulfation-sulfenylation. Zefirov, N.S.; Zyk, N.V.; Kutateladze, A.G.; Beloglazkina, E.K. Izv. Akad. Nauk SSSR, Ser. Khim. , 1991, (11), 2623.  [CA: 116, 105254c]
[CA: 116, 105254c]
[32]
Amino sulfenyl halides in synthesis of unsymmetrical diastereomeric sulfamato sulfides. Kutateladze, A.G.; Zyk, N.V.; Denisko, O.V.; Zefirov, N.S. Zh. Org. Khim. , 1991, 27, (3), 659.  [CA: 115, 159068t]
[CA: 115, 159068t]
[31]
Synthesis of thiophenes from acyl chloride - allyl chloride adducts. Mamedov, E.I.; Ismailov, A.G.; Zyk, N.V.; Kutateladze, A.G.; Zefirov, N.S. Sulfur Lett., 1991, 12, (3), 109.  [CA: 114, 185180x]
[CA: 114, 185180x]
[30]
Stereospecific sulfenylation of tetrafluorobenzobicyclo[2.2.2]octatriene (TFBBO). The structure of the ethyl sulfate of exo-12-phenylthio-3,4,5,6-tetrafluorotricyclo- [6.2.2.02,7]dodeca-2,4,6,9-tetraen-endo-11-ol. Zefirov, N.S.; Zyk, N.V.; Lapin, Yu.A.; Kutateladze, A.G.; Panov, V.N.; Goncharov, A.V.; Yufit, D.S.; Struchkov, Yu.T. Sulfur Lett., 1991, 12, (3), 103.  [CA: 114, 184863k]
[CA: 114, 184863k]
[29]
Difunctionalization of olefins with N-chloroamines in the presence of potassium pyrosulfate and bis(trimethylsilyl)sulfate. Zyk, N.V.; Mendeleeva, E.A.; Kutateladze, A.G.; Zefirov, N.S. Dokl. Acad. Nauk SSSR , 1990, 313, (5), 1138.  [CA: 114, 184850d]
[CA: 114, 184850d]
[28]
Vinylsulfenylation of olefins via sulfonate activation. Kutateladze, A.G.; Zyk, N.V.; Denisko, O.V.; Zefirov, N.S. Izv. Acad. Nauk SSSR, Ser. Khim. , 1990, (7), 1689.  [CA: 113, 211924u]
[CA: 113, 211924u]
[27]
Aminothiosulfonates - electrophilic synthones in the synthesis of thiosulfonates. Zefirov, N.S.; Zyk, N.V.; Kutateladze, A.G.; Butina, E.K. Zh. Org. Khim., 1990, 26, (10), 2225. 
[26]
Reaction of sulfenamides with olefins in the presence of potassium pyrosulfate. Zefirov, N.S.; Zyk, N.V.; Kutateladze, A.G.; Ignatchenko, E.S. Zh. Org. Khim. , 1990, 26, (3), 670.  [CA: 113, 151944p]
[CA: 113, 151944p]
[25]
Reaction of sulfenamides with cycloolefins in the presence of sulfamic acid, NH3-SO3. Zefirov, N.S.; Zyk, N.V.; Kutateladze, A.G.; Butina, E.K. Zh. Org. Khim., 1990, 26, (3), 671.  [CA: 113, 151945q]
[CA: 113, 151945q]
[24]
Sulfur trioxide mediated additon of N-chlorourethanes to carbon-carbon double bond. Zyk, N.V.; Mendeleeva, E.A.; Kolbasenko, S.I.; Kutateladze, A.G.; Zefirov, N.S. Izv. Acad. Nauk SSSR, Ser. Khim., 1989, (12), 2869.  [CA: 112, 216270s]
[CA: 112, 216270s]
[23]
Aminosulfenylation of olefins by sulfenamides in the presence of sulfamic acid, NH3-SO3. Zefirov, N.S.; Zyk, N.V.; Kutateladze, A.G.; Butina, E.K.; Vatsadze, S.Z. Izv. Acad. Nauk SSSR, Ser. Khim., 1989, (12), 2870.  [CA: 113, 5792w]
[CA: 113, 5792w]
[22]
Insertion reaction of N-sulfonylmethylamine into the sulfur-nitrogen bond of sulfenamides. Zefirov, N.S.; Zyk, N.V.; Kutateladze, A.G.; Ignatchenko, E.S.; Denisko, O.V. Zh. Org. Khim., 1989, 25, (7), 1576.  [CA: 112, 20730x]
[CA: 112, 20730x]
[21]
Sulfate-activated nitration of tetrafluorobenzobarrelene. Crystal and molecular structure of the ethyl sulfate derivative of anti-8-nitro-3,4-tetrafluorobenzobicyclo[3.2.1]octa-3,6-dien-exo-2-ol, C14H11F4NO6S. Zefirov, N.S.; Zyk, N.V; Lapin, Yu.A.; Kutateladze, A.G.; Panov, V.N.; Goncharov, A.V.; Kurkutova, E.N.; Struchkov, Yu.T. Dokl. Acad. Nauk SSSR, 1989, 307, (2), 378.  [CA: 112, 178297w]
[CA: 112, 178297w]
[20]
Phenylsulfenyl sulfate: a new sulfenylating reagent. Zyk, N.V.; Lapin, Yu.A.; Kutateladze, A.G.; Zefirov, N.S. Zh. Org. Khim., 1989, 25, (1), 198.  [CA: 111, 133716v]
[CA: 111, 133716v]
[19]
Aminosulfenylation of olefins by sulfenamides and thio-bis-amines in the presence of pyridine - sulfur trioxide. Rare example of stability of ?-sulfidoalcohol N,N-dimethylsulfamate. Zefirov, N.S.; Zyk, N.V.; Kutateladze, A.G.; Ugrak, B.I.; Struchkov, Yu.T.; Potekhin, K.A., Maleev, A.V. Dokl. Acad. Nauk SSSR , 1988, 301, (6), 1385.  [CA: 111, 6981]
[CA: 111, 6981]
[18]
Bifunctionalization of olefins by ethyl nitrate in the presence of sulfur trioxide. Zefirov, N.S.; Zyk, N.V.; Lapin, Yu.A.; Kutateladze, A.G. Sulfur Lett., 1988, 8, (3), 143.  [CA: 111, 153251t]
[CA: 111, 153251t]
[17]
Vicinal nitrato sulfates. Zyk, N.V.; Lapin, Yu.A.; Kutateladze, A.G.; Zefirov, N.S. Zh. Org. Khim. , 1988, 24, (4), 889.  [CA: 110, 23379j]
[CA: 110, 23379j]
[16]
Sulfonate-activated cleavage of the cyclopropane fragment of quadricyclane. Zyk, N.V.; Kolbasenko, S.I.; Kutateladze, A.G.; Lapin, Yu.A. Zh. Org. Khim., 1988, 24, (6), 1208.  [CA: 110, 134766c]
[CA: 110, 134766c]
[15]
Preparation of chlorobromohydrocarbons. Zefirov, N.S.; Zyk, N.V.; Kolbasenko, S.I.; Kutateladze, A.G.; Chizhov, A.O. U.S.S.R. Patent, SU-1351039, June 8, 1987, 
[14]
X-ray structural investigation of the addition product of thio-bis-morpholine to norbornene in the presence of the sulfur trioxide - pyridine complex. Zefirov, N.S.; Zyk, N.V.; Kutateladze, A.G.; Potekhin, K.A.; Struchkov, Yu.T. Sulfur Lett., 1987, 6, (5), 139.  [CA: 108, 112356a]
[CA: 108, 112356a]
[13]
Thiobis(amines) in the synthesis of thiobis(alkanol) disulfamates. Zefirov, N.S.; Zyk, N.V.; Kutateladze, A.G.; Lapin, Yu.A. Zh. Org. Khim., 1987, 23, (1), 229.  [CA: 107, 175541g]
[CA: 107, 175541g]
[12]
Sulfonate activation of N-chloroamines and sulfenamides by pyridine - sulfur trioxide in reactions with olefins. Zefirov, N.S.; Zyk, N.V.; Kutateladze, A.G.; Kolbasenko, S.I. Zh. Org. Khim., 1987, 23, (1), 227.  [CA: 107, 197597g]
[CA: 107, 197597g]
[11]
Electrophilic sulfamatosulfenylation of olefins. Zefirov, N.S.; Zyk, N.V.; Kutateladze, A.G.; Lapin, Yu.A. Zh. Org. Khim., 1987, 23, (2), 392.  [CA: 108, 37306d]
[CA: 108, 37306d]
[10]
Structure of 7-(2,4-Dinitrophenylthio)-exo-5-nitrobicyclo[2.2.1]hept-2-exo-yl Acetate. Sergeeva, M.V.; Yufit, D.S.; Struchkov, Yu.T.; Kurkutova, E.N.; Zefirov, N.S.; Zyk, N.V.; Kutateladze, A.G.; Kolbasenko, S.I. Acta Crystallogr., , 1986, C42, 1164.  [CA: 106, 93941c]
[CA: 106, 93941c]
[9]
Crystal and molecular structure of 3-(4-nitrophenylthio)-nortricyclane, C13H13NO2S. Potekhin, K.A.; Lindeman, S.B.; Struchkov, Yu.T.; Zyk, N.V.; Kutateladze, A.G.; Lapin, Yu.A.; Zefirov, N.S. Dokl. Acad. Nauk SSSR , 1986, 292, (4), 873.  [CA: 107, 175542h]
[CA: 107, 175542h]
[8]
Acid-catalized nucleophilic substitution of the N,N-diethylsulfamate group. Zefirov, N.S.; Zyk, N.V.; Kutateladze, A.G.; Lapin, Yu.A.; Chizhov, A.O. Zh. Org. Khim., 1986, 22, (11), 2462.  [CA: 107, 197570t]
[CA: 107, 197570t]
[7]
Crystal and molecular structure of exo-5-(4-nitrophenylthio)-endo-nortricyclene-3-ol N,N-dimethylsulfamate, C15H18N2O5S2. Potekhin, K.A.; Sedov, B.B.; Struchkov, Yu.T.; Zyk, N.V.; Kutateladze, A.G; Lapin, Yu.A.; Zefirov, N.S. Dokl. Acad. Nauk SSSR , 1986, 290, (3), 627.  [CA: 107, 39262q]
[CA: 107, 39262q]
[6]
New reaction: sulfamatosulfenylation of olefins. Zefirov, N.S.; Zyk, N.V.; Kutateladze, A.G.; Kolbasenko, S.I.; Lapin, Yu.A. Zh. Org. Chem., 1986, 22, (1), 214.  [CA: 106, 17940m]
[CA: 106, 17940m]
[5]
Sulfur Trioxide Assisted Electrophilic Addition of R2N-Cl to Olefins. Zefirov, N.S.; Zyk, N.V.; Kolbasenko, S.I.; Kutateladze, A.G. J. Org. Chem., 1985, 50, 4539. 
[4]
Sulfur trioxide activation of the addition of ethyl nitrite to olefins. Zefirov, N.S.; Zyk, N.V.; Kutateladze, A.G. Zh. Org. Khim. , 1984, 20, (11), 2473.  [CA: 102, 112911d]
[CA: 102, 112911d]
[3]
Reaction of chlorosulfamation of olefins. Zefirov, N.S.; Zyk, N.V.; Kolbasenko, S.I.; Kutateladze, A.G. Sulfur Lett. , 1984, 2, (3), 95.  [CA: 102, 5740a]
[CA: 102, 5740a]
[2]
Conjugate addition of NO2Cl to cycloolefins. Zyk, N.V.; Nikulin, A.V.; Kolbasenko, S.I.; Kutateladze, A.G.; Zefirov, N.S. Zh. Org. Khim. , 1984, 20, (6), 1329.  [CA: 102, 24131]
[CA: 102, 24131]
[1]
Reactions of N-chloroamines with olefins in the presence of sulfur trioxide. Zefirov, N.S.; Zyk, N.V.; Kolbasenko, S.I.; Kutateladze, A.G. Izv. Acad. Nauk SSSR, Ser. Khim. , 1984, (4), 959.  [CA: 101, 90449j]
[CA: 101, 90449j]



































 Angewandte's Hot Paper
Angewandte's Hot Paper 
 ACS Editors' Choice
ACS Editors' Choice 


 (Very Important Paper)
(Very Important Paper) 














 Featured Article
Featured Article 














 Featured Article. ACS Editors' Choice (made 20 most read JOC articles in August 2018)
Featured Article. ACS Editors' Choice (made 20 most read JOC articles in August 2018) 


 Featured Article. ACS Editors' Choice.
Featured Article. ACS Editors' Choice. 





































































 Angewandte's Hot Paper (click here)
Angewandte's Hot Paper (click here)
 Selected as an OBC HOT Article (click here)
Selected as an OBC HOT Article (click here)



















 esp@cenet
esp@cenet 

 esp@cenet
esp@cenet 








 Vol 13.
Vol 13. 






































 [CA: 117, 130842w]
[CA: 117, 130842w] 

 [CA: 116, 105254c]
[CA: 116, 105254c]  [CA: 115, 159068t]
[CA: 115, 159068t]  [CA: 114, 185180x]
[CA: 114, 185180x]  [CA: 114, 184863k]
[CA: 114, 184863k]  [CA: 114, 184850d]
[CA: 114, 184850d]  [CA: 113, 211924u]
[CA: 113, 211924u] 
 [CA: 113, 151944p]
[CA: 113, 151944p]  [CA: 113, 151945q]
[CA: 113, 151945q]  [CA: 112, 216270s]
[CA: 112, 216270s]  [CA: 113, 5792w]
[CA: 113, 5792w]  [CA: 112, 20730x]
[CA: 112, 20730x]  [CA: 112, 178297w]
[CA: 112, 178297w]  [CA: 111, 133716v]
[CA: 111, 133716v]  [CA: 111, 6981]
[CA: 111, 6981]  [CA: 111, 153251t]
[CA: 111, 153251t]  [CA: 110, 23379j]
[CA: 110, 23379j]  [CA: 110, 134766c]
[CA: 110, 134766c] 
 [CA: 108, 112356a]
[CA: 108, 112356a]  [CA: 107, 175541g]
[CA: 107, 175541g]  [CA: 107, 197597g]
[CA: 107, 197597g]  [CA: 108, 37306d]
[CA: 108, 37306d]  [CA: 106, 93941c]
[CA: 106, 93941c]  [CA: 107, 175542h]
[CA: 107, 175542h]  [CA: 107, 197570t]
[CA: 107, 197570t]  [CA: 107, 39262q]
[CA: 107, 39262q]  [CA: 106, 17940m]
[CA: 106, 17940m] 
 [CA: 102, 112911d]
[CA: 102, 112911d]  [CA: 102, 5740a]
[CA: 102, 5740a]  [CA: 102, 24131]
[CA: 102, 24131]  [CA: 101, 90449j]
[CA: 101, 90449j]